Abstract: Background: Oral malodor represents a common health concern affecting a substantial portion of the global population, the prevalence of which can range from 15% to 60%, highlighting its widespread occurrence. Bad breath, originating from pathogens in the oral cavity, can be mediated through treatment with antibacterial mouthwashes. This clinical trial explores the antibacterial effects and anti-malodor properties of a mouthwash containing both cetylpyridinium chloride and zinc lactate (CPC + Zn). Methods: In vitro antibacterial efficacy studies were run in the form of single-species short-interval kill tests on Streptococcus mutans and Aggregatibacter actinomycetemcomitans treated with CPC + Zn mouthwash, a placebo, and a negative control; a whole saliva bacterial kill test; and a biofilm viability test treated with CPC + Zn mouthwash, CPC alone mouthwash, essential oils with alcohol mouthwash, and essential oils alone mouthwash. A 3-week, double-blind, parallel clinical trial was also conducted in Chengdu, Sichuan, China, to evaluate the clinical efficacy of CPC + Zn mouthwash compared to a fluoride mouthwash for overnight oral malodor (12 hours post rinsing). Results: When CPC + Zn was tested against an A actinomycetemcomitans strain and S mutans strain, it gave a 7.11 (±0.549) and 8.83 (±0.405) log reduction in colony forming units (CFUs) relative to the phosphate buffered saline control, respectively, resulting in 99.9% reduction in bacterial load. Compared to a negative control, CPC + Zn mouthwash treatment significantly reduced bacterial biofilm viability 2 and 5 hours post treatment by 42.83% (P = .018) and 62.07% (P = .001), respectively. After 3 weeks of product use, the CPC + Zn test group exhibited a 33.5% decrease in oral malodor with a final baseline-adjusted mean score of 4.89 (±0.06; confidence interval 95% [4.76, 5.02]; P < .001). Conclusions: CPC + Zn mouthwash delivered superior antibacterial effects in both planktonic and biofilm cultures when compared to negative control in vitro and superior malodor reduction compared to control in vivo. The substantial reduction observed in both bacterial load and oral malodor suggests that the CPC + Zn mouthwash could serve as a highly effective oral hygiene product. The ability to maintain a “pleasant breath” status further enhances its applicability in daily oral care, improving users’ social interactions and overall quality of life. Practical Implications: Alcohol-free CPC + Zn mouthwash may be an effective treatment for prolonged oral malodor suppression through antibacterial properties.
Oral malodor, sometimes referred to as halitosis, represents a common oral condition affecting a substantial portion of the global population, the prevalence of which can range from 15% to 60%, highlighting its widespread occurrence.1 Bad breath, originating from pathogens in the oral cavity, can significantly influence an individual’s social interactions, as emotional well-being is negatively correlated with levels of total volatile sulfur compounds (VSCs) among other malodorous compounds.2 Bacterial degradation of amino acids from within the oral cavity results in the production of malodorous VSCs like hydrogen sulfide, methyl mercaptan, and dimethyl sulfide, the major contributors to bad breath.3 Multiple interventions have been assessed over the years for the control of oral malodor.4 Targeting the bacteria that cause bad breath is an important portion of those efforts because the bacteria that cause oral malodor are also implicated in periodontal disease.1
Cetylpyridinium chloride (CPC) has been a widely used antiseptic in mouthwashes and dentifrices since 1939.5 As a monocationic surfactant, its structure includes a positively charged hydrophilic pyridine head and a hydrophobic hexadecane tail. This amphiphilic nature allows it to interact with the oral environment and bacterial cell membranes. CPC’s antibacterial mechanism starts with an electrostatic interaction with negatively charged bacterial surfaces.6 The positive pyridine head displaces essential divalent cations on the membrane and the hydrophobic tail inserts into the lipid bilayer, disrupting membrane integrity and exhibiting broad-spectrum antibacterial activity.5 CPC’s surfactant properties ensure even distribution in the oral cavity.5 Clinical studies show CPC’s activity against oral pathogens linked to gingivitis and periodontal disease and its demonstrated bactericidal effects on biofilms.7 These multifaceted antibacterial mechanisms provide a strong rationale for the inclusion of CPC in mouthwash to combat VSC-producing bacteria.
Zinc ions, often in the form of zinc salts like zinc lactate, are common in anti-malodor mouthwashes8 and other oral hygiene products for benefits such as malodor reduction, plaque and calculus control, and antibacterial action.9 Zinc ions exhibit antibacterial activity against various oral bacteria,10 interfering with metabolic processes and reducing acid production by Streptococcus species.11 The addition of zinc lactate to mouthwash specifically shows long-term antibacterial effects.12 A key anti-malodor mechanism of zinc ions is their strong affinity for thiol groups within VSCs.8 Zinc ions interact with hydrogen sulfide to form insoluble, odorless zinc sulfide.8 In vitro studies show zinc salts can almost completely inhibit hydrogen sulfide volatilization.8 Clinical trials show zinc lactate mouthwashes reduce VSC concentrations and improve breath odor.9 The dual action of zinc lactate—antibacterial and VSC-neutralizing—makes it a valuable component in anti-malodor mouthwashes, potentially acting additively with CPC.
This clinical trial explores the antibacterial effects and anti-malodor properties of a mouthwash containing both CPC and zinc lactate. Reducing the oral bacterial load, especially anaerobic gram-negative bacteria on the tongue and in periodontal pockets, is key to managing oral malodor.13 This research provides an understanding of the potential additive nature between CPC and zinc lactate in combating oral malodor. A more effective anti-malodor mouthwash formulation could improve oral health and quality of life.
Materials and Methods
In vitro Antibacterial Efficacy Analysis
Single-Species Bacterial Kill Test
Treatments were as follows:
1. A test mouthwash containing 0.075% CPC and 0.28% zinc lactate in an alcohol-free base (CPC + Zn).
2. A matching placebo mouthwash.
3. A negative control containing phosphate buffered saline (PBS).
Single-species short-interval kill tests are a generally accepted measure of the antibacterial efficacy of liquid oral care formulations. Fine et al established a method using representative single species cultures of bacteria to enumerate the population of bacteria killed by a mouthwash formulation in a 30-second exposure, the recommended use time for most oral rinse formulas.14 In the present study, the authors employed a method similar to that of Fine et al using modifications previously reported in Schaeffer et al.15
Aggregatibacter actinomycetemcomitans (ATCC #43178) was grown from a single colony on a plate of brain heart infusion (BHI) agar with 10% sheep’s blood (Hardy Diagnostics, hardydiagnostics.com) by seeding a 30 mL culture of BHI broth supplemented with 1% sodium bicarbonate. Cultures were grown overnight at 37°C.
Streptococcus mutans (ATCC #25175) was grown from a single colony on a trypticase soy agar plate containing 5% sheep’s blood (Becton Dickinson, bd.com) by seeding a 30 ml culture of trypticase soy broth. Cultures were grown overnight at 37°C in a 5% carbon dioxide (CO2) atmosphere.
Briefly, 1 mL aliquots of an OD600~0.8 culture were harvested and treated for 30 seconds with the indicated mouthwash or control. Samples were pelleted and washed three times with sterile PBS to remove the treatment. Washed pellets were resuspended in 1 ml of sterile PBS, serially diluted and plated on agar plates for colony enumeration. A actinomycetemcomitans samples were plated on BHI agar containing 5% defibrinated sheep’s blood. Plates were incubated for 48 to 72 hours in a semi-anaerobic atmosphere prior to counting colonies. S mutans samples were plated on tryptic soy broth agar plates containing 5% defibrinated sheep’s blood and incubated in a 5% CO2 atmosphere for 24 to 48 hours prior to counting.
Colony counts were used to determine the numbers of viable bacteria per mL sample (CFU/mL), and this value was used to determine the log reduction in CFUs relative to the PBS-treated samples.
Whole Saliva Bacterial Kill Test
In order to validate the efficacy of formulas against a more realistic, robust bacterial population, the authors harvested whole saliva from a single, healthy adult volunteer via expectoration. The volunteer provided unstimulated saliva after having abstained from eating, drinking, and all oral hygiene for at least 8 hours.
Whole saliva was aliquoted into 200 μL samples in individual sterile microfuge tubes. Aliquots of saliva were treated 1:1 with the indicated mouthwash and allowed to incubate for 30 seconds. Following the 30-second treatment, exposure was disrupted by adding 1 mL of sterile Dey-Engley neutralization broth (D/E broth) to each sample. This medium contains a mix of thiols and detergents capable of neutralizing a wide range of antimicrobial agents, such as quaternary ammonium compounds, metals, and surfactants.
Samples were pelleted by centrifugation and then washed one time by resuspending in 1 mL of sterile PBS and centrifuging again. Washed samples were resuspended in a fresh 1 mL aliquot of sterile PBS. Two hundred μL of each sample was transferred in duplicate to a sterile 96-well plate, and serial 10-fold dilutions were performed in sterile PBS. One hundred μL of relevant dilutions was plated on trypticase soy agar plates supplemented with 5% sheep’s blood. Plates were incubated for 24 hours at 37℃ in a 5% CO2 atmosphere. Colony counts were obtained from relevant plates. Data are reported as a reduction in CFUs/mL relative to a negative control sample treated with sterile PBS.
Biofilm Viability Assay
Treatments were as follows:
1. A test mouthwash containing 0.075% CPC, 0.28% zinc lactate, and 0.05% sodium fluoride in an alcohol-free base (CPC + Zn).
2. A commercially available mouthwash containing 0.075% CPC in an alcohol-free base (CPC) (Colgate-Palmolive Co., colgatepalmolive.com).
3. A commercially available mouthwash containing essential oils and 21.6% ethanol (EO + EtOH) (Johnson & Johnson, jnj.com).
4. A commercially available mouthwash containing essential oils and no alcohol (EO) (Johnson & Johnson).
5. A negative control containing PBS.
Laboratory biofilms used in this study were cultured from whole saliva collected from unbrushed donors following Institutional Review Board approval. The donors were asked to refrain from eating, drinking, and oral hygiene 12 hours prior to saliva donation. Before collection, the donors were provided with an unused toothbrush and instructed to brush their teeth without toothpaste for 1 minute in order to dislodge plaque from tooth surfaces. They were instructed to proceed to spit in a sterile 50 mL conical tube until a total volume of 10 mL was reached. The whole saliva was then vortexed to homogenize the samples prior to the addition of 40 mL McBain medium supplemented with 5 µg/mL hemin (final concentration; ThermoFisher, thermofisher.com) and 1 µg/mL menadione (final concentration; ThermoFisher). The resulting bacterial suspension was distributed in 1.5 mL aliquots into a sterile 24-well polystyrene plate. The biofilms were cultured on hydroxyapatite disks for 24 hours at 37℃ under an environment containing 5% CO2. Media was replaced twice daily thereafter and biofilms were cultured as described for an additional 48 hours. Biofilms were treated with undiluted mouthwashes for 30 seconds at room temperature on an elliptical shaker at the rate of 90 revolutions per minute. After treatment, the hydroxyapatite disks with the cultured biofilms were rinsed by dipping five times in sterile deionized water for a total of two rounds. This wash step was carried out in sterile 24-well polystyrene plates containing 1.7 mL of sterile deionized water. After washing, the biofilms were allowed to recover in sterile deionized water at 37℃ under 5% CO2 for 2 and 5 hours. To collect the biofilms for viability measurement, the treated biofilms were sonicated for a total of 2 minutes at 30-second intervals per side. Biofilm viability was measured through the quantification of total ATP (adenosine 5’-triphosphate) using BacTiter-Glo™ Microbial Cell Viability kit (Promega, promega.com). The test reagents were prepared as described by the manufacturer with 100 μL of the ATP reagent added to 50 µL of bacterial suspension. The reaction was incubated at room temperature in the dark for no more than 5 minutes. Total ATP was measured through luminometry with values expressed at relative light units (RLUs). Additionally, RLUs were normalized versus total bacterial mass as determined through Syto 9 staining (ThermoFisher) with the control group set to “1.” Percent reduction in biofilm viability was calculated relative to the control. The experiment was performed a total of four times. Statistical significance was determined using Tukey’s multiple comparison test.
Clinical Malodor Analysis
Interventions
1. A test mouthwash containing 0.075% CPC, 0.28% zinc lactate, and 0.05% sodium fluoride in an alcohol-free base (CPC + Zn).
2. A commercially available mouthwash with 100 ppm fluoride (F) (Colgate-Palmolive Co.).
Study Design
This 3-week, double-blind, parallel clinical trial was conducted in Chengdu, Sichuan, China, to evaluate the clinical efficacy of CPC + Zn mouthwash compared to F mouthwash for overnight oral malodor (12 hours post rinsing). Qualifying participants were randomly assigned via a computer-generated random number list to either the CPC + Zn test group or the F control group in such a way that examiner, subjects, and statistician were blind to allocation. All products were concealed and coded by the sponsor to preserve blinding.
Participants and Inclusion/Exclusion Criteria
Eighty healthy adults, male and female, 18 through 70 years old were recruited and divided into two groups of 40 participants per group. For inclusion in the study, participants must have aligned with the following inclusion criteria: availability for the 3-week duration, initial mean organoleptic oral malodor score ≥6.0 and ≤8.4, and willingness to sign informed consent form. Individuals were excluded from this study if they had orthodontic bands, ≥1 tumor of the soft or hard tissue within the oral cavity, advanced periodontal disease, ≥5 carious lesions requiring restorative treatment, use of antibiotics or participation in another clinical study within 1 month of this study, received dental prophylaxis within 2 weeks of baseline examination, taken prescription medications that may interfere with the study outcome, existing medical condition prohibiting the individual from eating or drinking for up to 4 hours, history of allergies to oral care products, history of drug or alcohol abuse, or self-reported pregnancy or lactation.
Study Protocol
All qualifying participants were provided with their group-specific mouthwash, a regular fluoride toothpaste containing 0.76% sodium monofluorophosphate, and a manual toothbrush. Participants were asked to brush their teeth for 2 minutes with the provided toothpaste and toothbrush once in the morning and once in the evening. All rinsing followed product-specific per label instructions. Following each instance of brushing, the CPC + Zn test group members were asked to rinse for 30 seconds with 20 mL of assigned mouthwash without rinsing with water afterwards, and the F control group members were asked to rinse for 1 minute with 10 mL of assigned mouthwash without rinsing with water afterwards as per label mouthwash instructions.
Oral malodor evaluations were conducted by four trained judges using a nine-point hedonic scale from most unpleasant (1) to most pleasant (9). Baseline evaluations were conducted in the morning after participants refrained from eating, drinking, and all oral hygiene, including brushing, rinsing, and flossing for at least 6 hours. Participants were then evaluated after following the oral care regimen for 3 weeks. This evaluation was conducted 12 hours post rinsing (overnight).
Statistical Analysis
Participant group size was determined as about 40 for a significance with 80% power. Subject-wise baseline oral malodor scores were determined by taking the mean of the scores provided by all four judges for each subject. Baseline and week 3 group scores were determined by calculating the mean of all scores within each group. Paired t-test was used to compare the within-treatment, week 3 scores to baseline scores. Between-treatment comparison was performed with analysis of covariance baseline-adjusted week 3 means and baseline as the covariate. All tests were two-sided with a 0.05 significance level.
Results
In vitro Antibacterial Efficacy
Single-Species Bacterial Kill Test
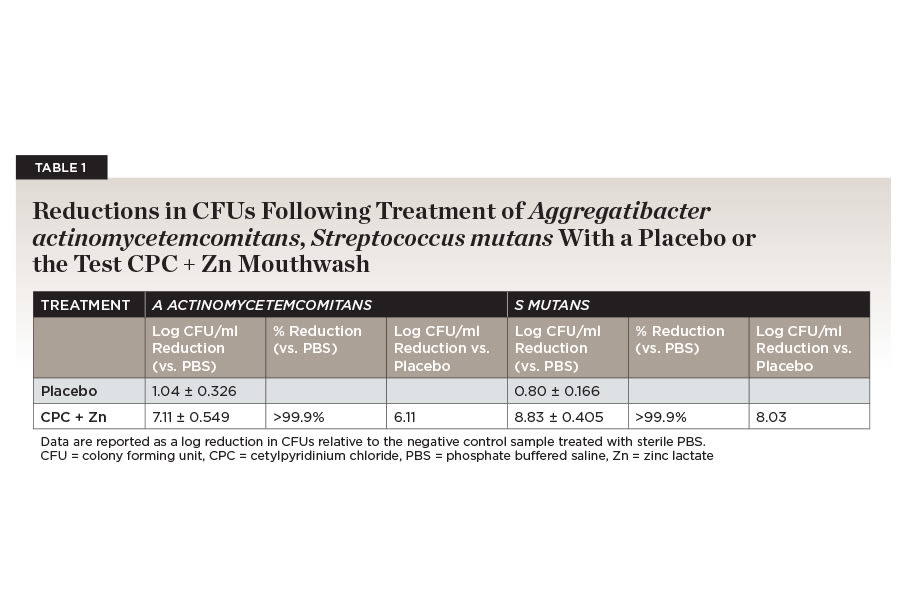
Simple in vitro bacterial kill studies are a useful tool for enumerating the ability of a formula to kill target bacteria. The treatment of bacterial samples with the placebo formula without CPC or other active ingredients resulted in a small (≤1 log) but consistent reduction in CFUs for both strains due to the presence of excipients such as surfactants. The placebo treatment reduced S mutans counts by 0.80 (±0.166) log CFUs/mL and A actinomycetemcomitans by 1.04 (±0.326) log CFUs/mL. Due to these clear differences in the performance of the placebo formula, the performance of the test mouthwashes with CPC + Zn was reported relative to the colony counts obtained following treatment with both placebo and the negative control mouthwash to help distinguish formula effects from active ingredient effects.
When CPC + Zn was tested against an A actinomycetemcomitans strain, it gave a 7.11 (±0.549) log reduction in CFUs relative to the PBS control. This represented a >6 log increase beyond the placebo formula. CPC + Zn treatment resulted in statistically significant greater reduction (P < .0001) in planktonic A actinomycetemcomitans than the negative control. CPC + Zn reduced planktonic A actinomycetemcomitans by >99.9% over a negative control formula (Table 1).
CPC + Zn was also tested against planktonic S mutans. CPC + Zn gave a reduction of 8.83 (±0.405) log CFUs compared to the PBS-treated negative control. This was >8 logs greater reduction in CFUs than the matched placebo formula. The test formula was statistically significantly (P < .0001) different from the negative control. Both formulas gave reductions in planktonic S mutans that were >99.9% greater than the negative control formulas (Table 1).
Whole Saliva Bacterial Kill Test
While single-species kill assays are a valuable tool for quantifying the impact of oral care products on individual species of bacteria found in the oral cavity, these assays are not reflective of the way in which bacteria exist in patients’ mouths. Therefore, a whole saliva short exposure test was used to validate the in vitro performance of formulations.
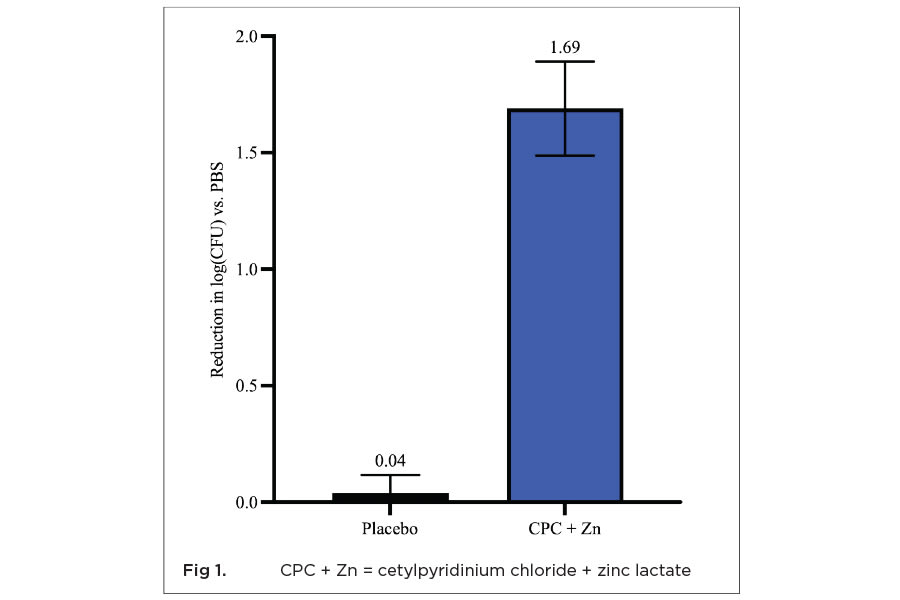
This method is used to measure the immediate impact of typical use time treatments with the indicated formula on the viability of planktonic bacteria, represented by whole saliva. The CPC + Zn mouthwash gave a 1.69 log reduction (>90%) in total salivary bacterial counts (Figure 1). For comparison purposes, a matched placebo mouthwash with no CPC or Zn gave only a 0.04 log reduction in CFUs/ml with the same treatment. This difference in killing was statistically significantly (P = .0002) greater than the placebo.
Biofilm Viability
Compared to the negative control, treatment with the CPC + Zn mouthwash showed statistically significant reduction in biofilm viability 2 and 5 hours post treatment by 42.83% (P = .018) and 62.07% (P = .001), respectively. CPC mouthwash reduced 4.74% (P > .999) and 40.24% (P = .079) biofilm viability 2 and 5 hours post treatment, respectively, but the reduction was not significant compared to the negative control. Mouthwash containing EO + EtOH reduced 42.61% (P = .103) and 19.46% (P = .861) biofilm viability 2 and 5 hours post treatment, respectively, but it was not statistically significant compared to the negative control. Mouthwash containing essential oils and no alcohol reduced biofilm viability by 14.25% (P = .987) after 2 hours and showed similar viability to the negative control 5 hours after treatment (P > .999). CPC-containing mouthwashes were the only treatments that continued to suppress bacterial biofilm viability over time; however, CPC + Zn was the only treatment that showed significant reduction at both timepoints compared to the negative control (Figure 2).
Clinical Malodor Reduction
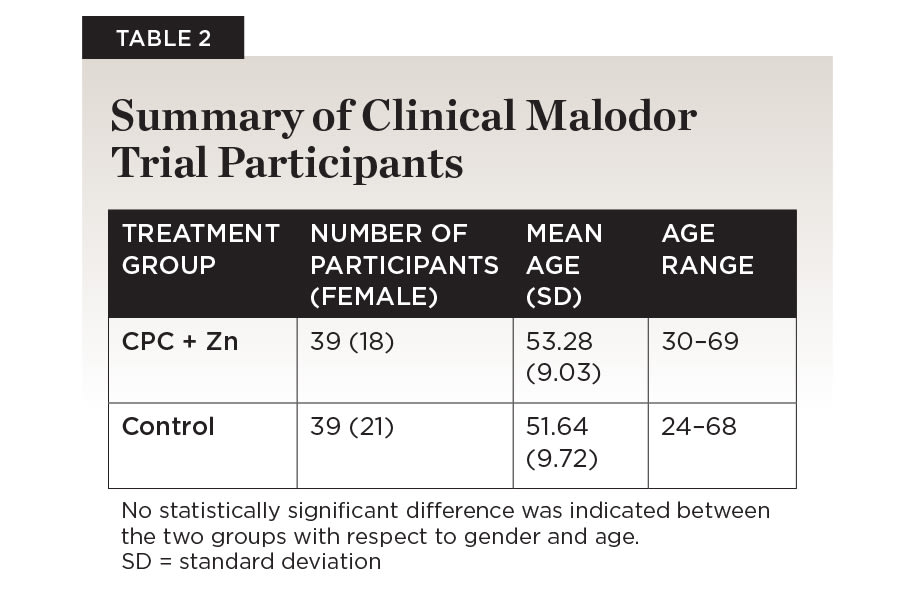
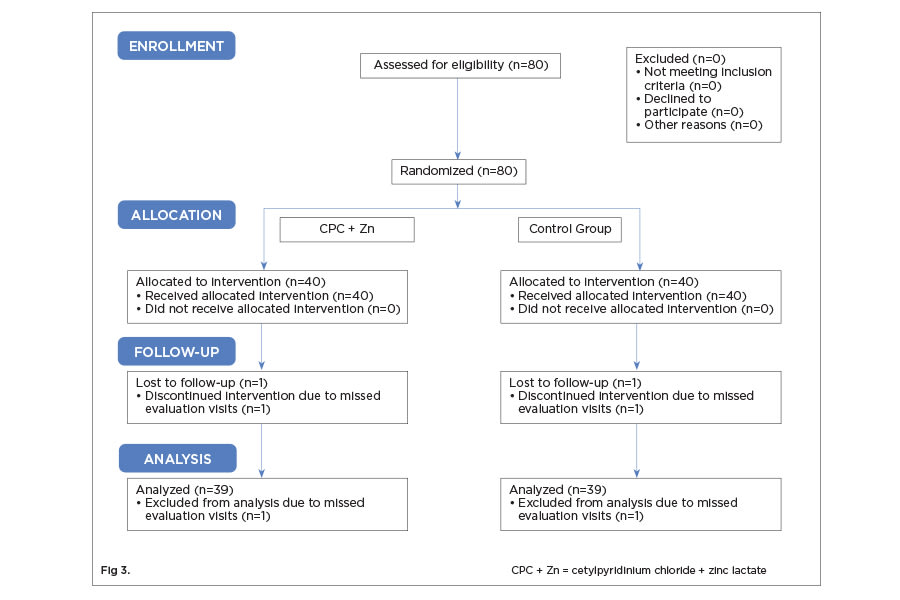
Eighty individuals were recruited based on inclusion criteria to participate in this study. A total of 78 participants completed the study. One person per group was dropped from analysis because they failed to make the final appointment (Figure 3). There were no statistically significant differences between the two treatment groups with respect to gender (P = .497) and age (P = .442) (Table 2). No adverse events were recorded, including hard and soft tissue examination results.
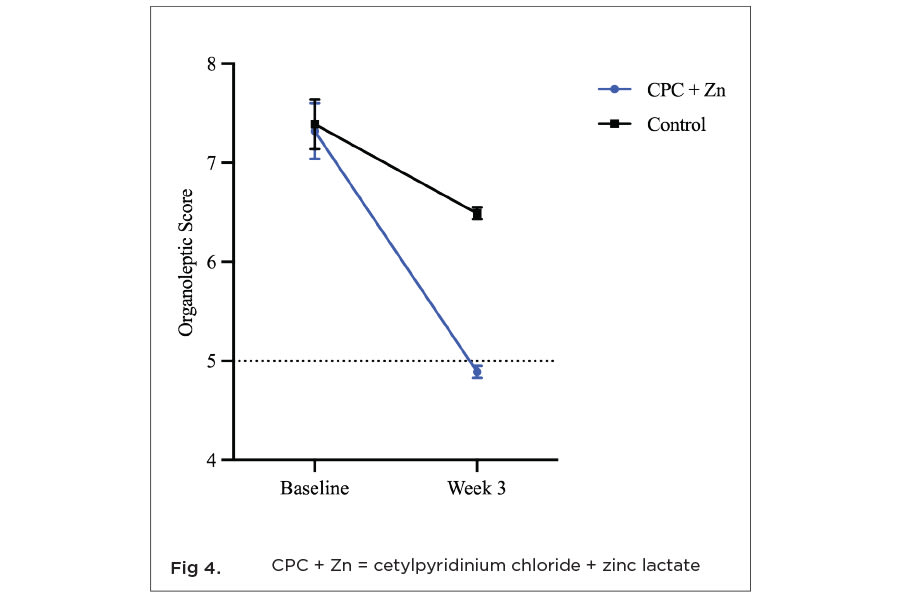
There was no statistically significant (P = .244) difference between baseline organoleptic scores for each treatment group. The CPC + Zn test group had a mean baseline score of 7.32 (±0.28; confidence interval [CI] 95% [7.09, 7.68]). The control group had a mean baseline score of 7.39 (±0.25; CI 95% [7.19, 7.71]). Both mean scores represent breath considered to be between moderately unpleasant (7) and very unpleasant (8). After 3 weeks of product use, both treatments showed statistically significant (P < .05) reduction in malodor compared to baseline. The CPC + Zn test group exhibited a 33.5% decrease in oral malodor with a final baseline-adjusted mean score of 4.89 (±0.06; CI 95% [4.76, 5.02]; P < .001) (Figure 4). This mean score is considered to represent breath quality between neutral (5) and slightly pleasant breath (4). The control group had a 12.0% reduction in oral malodor with a baseline-adjusted mean organoleptic score of 6.49 (±0.06; CI 95% [6.36, 6.62]; P < .001), which is considered to be breath quality between slightly unpleasant (6) and moderately unpleasant (7). The CPC + Zn group had a 24.7% greater reduction in oral malodor than the control group.
Discussion
These series of in vitro and in vivo studies demonstrated the potent antibacterial and anti-malodor effects of a CPC + Zn mouthwash, achieving significant reductions in bacterial presence across various environments. The >99.9% reduction in planktonic single-species bacteria and >90% reduction of salivary bacteria showcased the mouthwash’s formidable antibacterial capacity. In biofilm, CPC + Zn mouthwash was the only treatment that showed significant and compounding reduction in bacterial biofilm as time increased compared to the negative control. While the EO + EtOH mouthwash was observed to have a similar 43% reduction in biofilm viability as the CPC + Zn mouthwash at 2 hours, the antibacterial efficacy of EO + EtOH declined after 5 hours, whereas the antibacterial efficacy of CPC + Zn increased over time. Notably, the continued significant suppression of bacterial biofilm viability over time corroborates the mouthwash’s efficacy in improving breath quality in clinical trials. The transition from the “unpleasant” to the “pleasant” breath range in organoleptic scores after 3 weeks of use illustrated CPC + Zn’s tangible benefits in a daily-use scenario.
Previous studies with CPC-based mouthwashes reflect the ingredient’s antibacterial properties. A similar in vitro single-species kill study on A actinomycetemcomitans and S mutans performed with two different mouthwashes containing 0.075% CPC and 0.05% sodium fluoride also showed a >99.9% reduction in each species.15 A biofilm study performed with confocal laser scanning microscopy and fluorometric analyses observed that treatment with an alcohol-free 0.075% CPC-containing mouthwash showed a significantly increased number of damaged biofilm cells compared to placebo mouthwash.15,16 Additionally, oral care formulations have been enhanced with the addition of zinc salt in previous studies, showing that zinc acts in a compounding manner to antimicrobial properties.12,17,18 A clinical study featuring a mouthwash formula containing 0.075% CPC and 0.28% zinc lactate versus mouthwash with 0.075% CPC found that the addition of zinc lactate to the formula significantly enhanced the antiplaque and antigingivitis efficacy of the mouthwash.19 These results combined suggest that the antibacterial properties seen in the CPC + Zn mouthwash here may be driven by the 0.075% CPC and further strengthened by the zinc lactate.
In addition to its broad spectrum antibacterial properties, CPC has also been shown to specifically suppress the expression of genes related to VSC production in anaerobic pathogens in single-species studies,20 indicating that CPC acts in conjunction with zinc lactate’s well-known VSC neutralization8 to provide the malodor protection seen in the clinical trial performed here. Supporting this, a previous clinical trial where participants brushed with a regular fluoride toothpaste and rinsed with a 0.075% CPC mouthwash showed significant reduction in VSC concentrations in addition to significant improvement in oral malodor compared to brushing alone.21
Conclusion
The substantial reduction observed in both bacterial load and oral malodor suggests that the CPC + Zn mouthwash serves as a highly effective oral hygiene product, combatting oral biofilms and targeting malodor. The ability to provide “pleasant breath” further enhances its applicability in daily oral care, improving users’ social interactions and overall quality of life. These findings underscore the potential for this mouthwash formulation to become a preferred option in oral health regimens, particularly for individuals seeking enhanced antibacterial and breath-freshening benefits beyond conventional products.
ACKNOWLEDGMENTS
Technical writing and data visualization were provided by Meghan A. Berryman, PhD. The author contributions were as follows: LS and CD: investigation, formal analysis; RA: methodology; LM: formal analysis, validation; NL and YZ: conceptualization, funding acquisition, supervision; DH: project administration, investigation. All authors contributed to writing, review, and editing.
DISCLOSURES
This clinical trial was supported by funding from Colgate-Palmolive Company. The study was reviewed and approved by the Institutional Review Board of China Oral Health Foundation, 18-A South Avenue, Zhongguancun, Haidian District Beijing, 100081.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
ABOUT THE AUTHORS
Lyndsay Schaeffer, PhD
Director of Oral Microbiome and Microbiology, Colgate-Palmolive Co., Piscataway, New Jersey
Carlo Amorin Daep, PhD
Senior Researcher, Colgate-Palmolive Co., Piscataway, New Jersey
Rabab Ahmed, PhD
Technical Associate, Colgate-Palmolive Co., Piscataway, New Jersey
Luis R. Mateo, MA
President, LRM Statistical Consulting LLC, West Orange, New Jersey
Nicky Li, DMD, MPH
Former Technical Associate, Colgate-Palmolive Co., Piscataway, New Jersey
Deyu Hu, DDS, MS
Professor, West China Dental Institute of Chengdu
Yun-Po Zhang, PhD, DDS (Hon)
Senior Vice President and Distinguished Fellow, Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey