Abstract: Background: Antibacterial mouthwashes are an effective method for reducing plaque and gingivitis when used regularly as part of an oral hygiene regimen that includes brushing and flossing. However, mouthwashes formulated with high ethanol content can be associated with a burning sensation that typically leads to lack of compliance. Alcohol-free, antibacterial mouthwash may be an effective alternative for antiplaque and antigingivitis treatment without the burn. Methods: A 118-participant, phase III, randomized, single-center, three-arm, examiner-blind, parallel-group clinical trial was conducted over 6 weeks in the Dominican Republic to assess the efficacy of a 0.075% cetylpyridinium chloride (CPC), 0.28% zinc lactate, and 0.05% sodium fluoride mouthwash in an alcohol-free base (CPC + Zn), an essential oils and 21.6% ethanol mouthwash (EO + EtOH), and a placebo mouthwash on established dental plaque and gingivitis. Scoring indices were used to measure whole mouth, interproximal, and severity for both plaque and gingivitis. Results: All plaque and gingivitis scores improved statistically significantly for both the CPC + Zn (all: P < .001) and EO + EtOH (all: P < .001) treatment groups for all timepoints compared to baseline. After 6 weeks, the CPC + Zn group exhibited a 37.2% reduction in plaque severity and a 47.7% reduction in gingivitis severity (P < .001), and the EO + EtOH group had a 35.9% reduction in plaque severity and a 38.6% reduction in gingivitis severity compared to baseline (P < .001). There was no statistically significant (P > .05) difference between the impact that CPC + Zn and EO + EtOH had on plaque and gingivitis reduction for all scores measured. Conclusions: These results demonstrated parity between CPC + Zn and EO + EtOH mouthwash formulas in the reduction of dental plaque and gingivitis. Both treatments significantly reduced whole-mouth, interproximal, and severity scores compared to the placebo. Practical Implications: Alcohol-free CPC + Zn mouthwash may be an effective alternative to control plaque and gingivitis for patients who struggle to comply with a regimen with alcohol-containing mouthwashes.
Mouthwash plays an important role in the reduction of plaque biofilm and the management of oral disease.1 While toothbrushing continues to be the predominant recommendation for mechanical removal of supragingival plaque, interproximal plaque remains intact on the tooth surface after brushing alone with regular toothbrushes.2 Antibacterial mouthwashes have proven to be an effective method for reducing interproximal plaque and whole-mouth gingivitis in adjunct use with toothbrushing.3-5 However, many commercially available mouthwashes are formulated with a high ethanol content, which is associated with intense oral pain and lower user compliance.6-8 Alcohol-free, antibacterial mouthwash may be an effective alternative for antiplaque and antigingivitis reduction without the burn.
Cetylpyridinium chloride (CPC) is a quaternary ammonium compound widely used for its antibacterial properties across several types of dental treatments. It is recognized as safe and effective for use against plaque and gingivitis in the US Food and Drug Administration’s 2003 Advance Notice of Proposed Rulemaking for Over-the-Counter Antigingivitis/Antiplaque Drug products, based on the recommendation of the Dental Plaque Subcommittee of the Nonprescription Drugs Advisory Committee.9,10 The amphiphilic nature of the CPC compound disrupts and destroys bacterial cell membranes, allowing for the cellular remnants to be rinsed off the oral surface.11 A recent review investigating eight randomized clinical trials featuring CPC-containing mouthwashes found that CPC demonstrated significant and superior antiplaque efficacy across all studies compared to the controls.3
The mouthwash formula presented in this study contained 0.075% CPC and 0.28% zinc lactate (CPC + Zn). In vitro studies found that CPC + Zn reduced periodontal pathogens while allowing for the colonization of oral health–associated bacterial species in biofilm.12 The addition of zinc lactate to the formula also increases its efficacy in vivo, as evidenced by a 54.5% greater reduction in gingivitis severity after 6 weeks of use compared to a mouthwash formula containing 0.07% CPC alone.13 Zinc has been shown to have antimicrobial properties in the context of halitosis-associated bacteria and can be clinically effective against halitosis in a mouthwash.14,15 CPC + Zn has also been shown to have a 44.8% greater reduction in plaque compared to an alcohol-free essential oil mouthwash.16
Mouthwashes with essential oils in an ethanol base (EO + EtOH) are among the most widely studied mouthwashes due to their antimicrobial properties and effective plaque control.17 A systematic review found that EO + EtOH significantly reduced plaque and gingivitis compared to a CPC mouthwash without zinc lactate.18 A recent clinical trial even indicates that EO + EtOH achieved a more thorough reduction in interproximal plaque than flossing as an adjunct to brushing.19
The present clinical trial was designed to evaluate the antiplaque and antigingivitis efficacy of a CPC + Zn mouthwash compared to an EO + EtOH mouthwash and a placebo mouthwash with no active ingredients. The authors hypothesized that both CPC + Zn and EO + EtOH will equally reduce plaque and gingivitis significantly compared to the placebo.
Materials and Methods
Interventions
1. A test mouthwash containing 0.075% CPC, 0.28% zinc lactate, and 0.05% sodium fluoride in an alcohol-free base (CPC + Zn) (Colgate-Palmolive Co., colgatepalmolive.com).
2. A commercially available mouthwash containing essential oils and 21.6% ethanol (EO + EtOH) (Johnson & Johnson, jnj.com).
3. An alcohol-free placebo mouthwash with 0.05% sodium fluoride.
Study Design
This phase III, randomized, single-center, three-arm, examiner-blind, parallel-group clinical trial was conducted in the Santo Domingo, Dominican Republic, area to assess the clinical efficacy of an alcohol-free CPC + Zn mouthwash compared to an EO + EtOH mouthwash and a negative control mouthwash without CPC in a population with established dental plaque and gingivitis over a 6-week period.
Qualifying participants were randomly assigned to one of three treatment groups in such a way that neither the examiner nor the statistician was aware of the identity of the product allocation. Participants were assigned an identification number in chronological order from 001 to 120. They were then randomized to a study group by a computer-generated randomization list.
All products were concealed with white overwrapped paper. Label information was limited to a mouthwash code, instructions for at-home use, and safety information. The examiner, study site personnel, and statistician were blinded to product assignment.
Participants and Inclusion Criteria: A total of 120 healthy female and male adults between the ages of 18 and 70 were recruited from the Santo Domingo, Dominican Republic, area and randomized equally into three groups of 40 participants. To be included in the study, participants were required to be available for the 6-week duration, had to be considered in good health by the practitioner, and had to have ≥20 uncrowned permanent natural teeth, excluding the third molars. Upon baseline inspection, participants were required to have a Löe-Silness Gingival Index score of ≥1.0 and a Turesky modification of the Quigley-Hein Plaque Index score of ≥1.5.
Exclusion criteria included the presence of orthodontic bands, partial removable dentures, tumors of the soft or hard tissue in the oral cavity, advanced periodontal disease, or ≥5 decayed carious lesions requiring restorative treatment. Individuals were also excluded if they were pregnant or lactating women, had received dental prophylaxis 2 weeks prior to entry into the study, had participated in a clinical study within 1 month of this study, had a history of allergies to oral care products or their ingredients, had an existing medical condition that prevented eating or drinking for periods up to 4 hours, had a history of alcohol or drug use, or were prescribed any medication that may interfere with the study outcome.
Study Protocol
In addition to study mouthwash, all qualifying participants were provided with a regular fluoride toothpaste containing 0.76% sodium monofluorophosphate and a manual soft-bristled toothbrush. Participants were instructed to brush in the morning and in the evening for 1 minute with approximately 1.5 g of the provided toothpaste on the provided toothbrush. Following each instance of brushing, participants were instructed to rinse for 30 seconds with 20 mL of their assigned mouthwash for 6 weeks.
Participants were assessed for dental plaque according to the Turesky modification of the Quigley-Hein Plaque Index.20,21 Dentition of each tooth was disclosed and plaque was scored at the distofacial (DF), midfacial (MidF), mesiofacial (MF), distolingual (DL), midlingual (MidL), and mesiolingual (ML) surfaces. The scoring system criteria were: 0 = no plaque; 1 = separate flecks of plaque at the cervical margin; 2 = thin, continuous ≤1 mm plaque band at the cervical margin; 3 = plaque band with >1 mm width covering < one third of the crown of the tooth; 4 = ≥ one third and < two thirds plaque coverage on the crown of the tooth, 5 = ≥ two thirds plaque coverage on the crown of the tooth.
Whole-mouth plaque score = [(DF + MF + MidF) + (DL + ML + MidL)] / 6
Interproximal plaque score = [(DF + MF) + (DL + ML)] / 4
Plaque severity score = total number of 3–5 scores / 6
Participants were assessed for gingival inflammation and scored at the DF, MidF, MF, DL, MidL, and ML sites of each tooth according to the Löe-Silness Gingival Index.22,23 The criteria for scoring were: 0 = no inflammation; 1 = slight change in color and texture, indicating mild inflammation; 2 = moderate glazing, redness, edema, and hypertrophy, indicating moderate inflammation; 3 = marked redness and spontaneous bleeding, indicating severe inflammation.
Whole-mouth gingivitis score = [(DF + MF + MidF) + (DL + ML + MidL)] / 6
Interproximal gingivitis score = [(DF + MF) + (DL + ML)] / 4
Gingivitis severity score = total number of 2–3 scores / 6
Participants were also assessed by the dental examiner visually with a dental light and mirror to evaluate the soft and hard palate, gingival mucosa, buccal mucosa, mucogingival fold areas, tongue, sublingual and submandibular areas, salivary glands, tonsillar and pharyngeal areas, and teeth.
Adverse events were monitored via participant interview and dental examination.
Statistical Analysis
A sample size of 120 participants divided into three groups of 40 participants was based on a response measure of 0.58, a significance level of α = 0.05, a 10% attrition rate, and an 80% power level as previously described in the literature.24,25 The per protocol population was analyzed. Chi-square analysis was used to determine significant differences for gender between groups. An independent t-test was used to determine significant differences for age between groups.
Statistical analyses were performed separately for scores from Löe-Silness Gingival Indices and Quigley-Hein Plaque Indices assessments. Analysis of variance (ANOVA) was performed for between-treatment baseline comparisons. Paired t-tests were used for within-treatment baseline versus follow-up score comparisons. Analysis of covariance (ANCOVA) was performed for within-treatment baseline-adjusted versus follow-up score comparisons. Tukey’s test for multiple comparisons was used to conduct post-ANCOVA pair-wise comparisons of treatment groups. All statistical tests conducted used a significance of α = 0.05 and were two-sided.
Results
Trial Participants
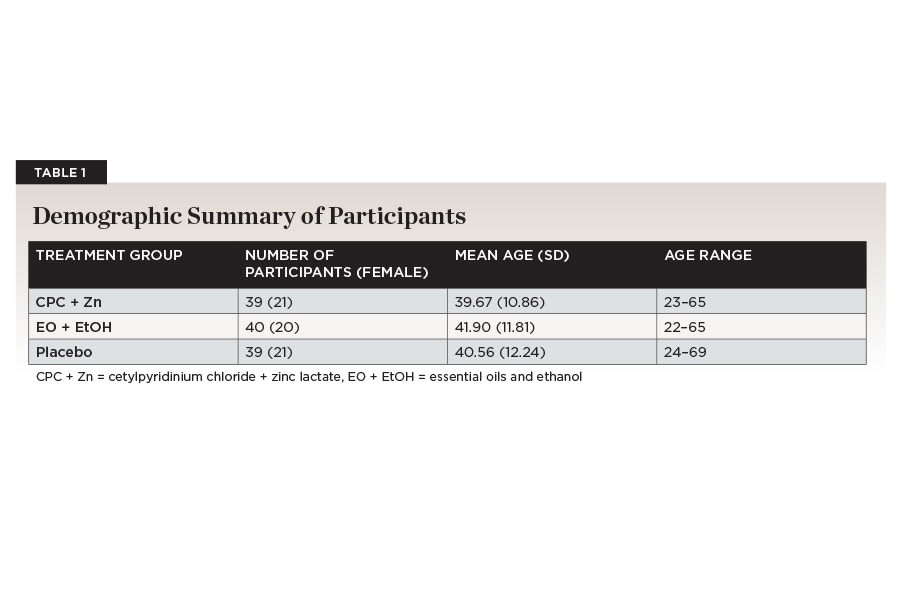
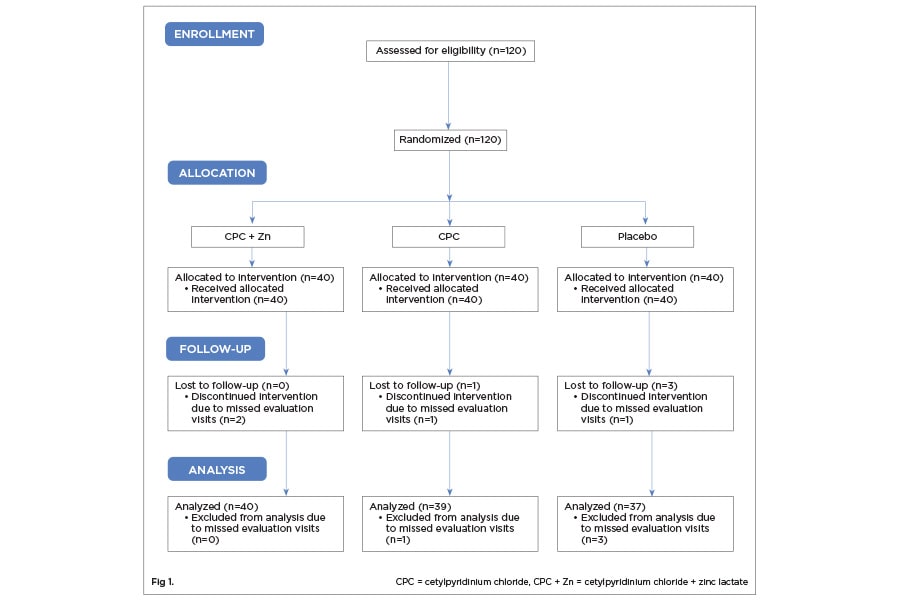
One-hundred-twenty participants were accepted into the study based on the inclusion and exclusion criteria (Figure 1). Two participants were dismissed for not complying with protocol requirements. One-hundred-eighteen participants were randomized into the CPC + Zn treatment group (n = 39; female = 21), the EO + EtOH treatment group (n = 40; female = 20), and the placebo group (n = 39; female = 21). The age range for the CPC + Zn group was 23 to 65 (mean ± standard deviation [SD]: 39.67 ± 10.86). The age range for the EO + EtOH group was 22 to 65 (41.90 ± 11.81). The age range for the placebo group was 24 to 69 (40.56 ± 12.24). There were no statistically significant differences between gender (P = .925) or age (P = .693) across the three treatment groups (Table 1).
Neither examiner nor participants reported any adverse effects on the oral hard or soft tissue. The two participants who did not complete the study reported reasons unrelated to the treatments.
Within-Treatment Analysis
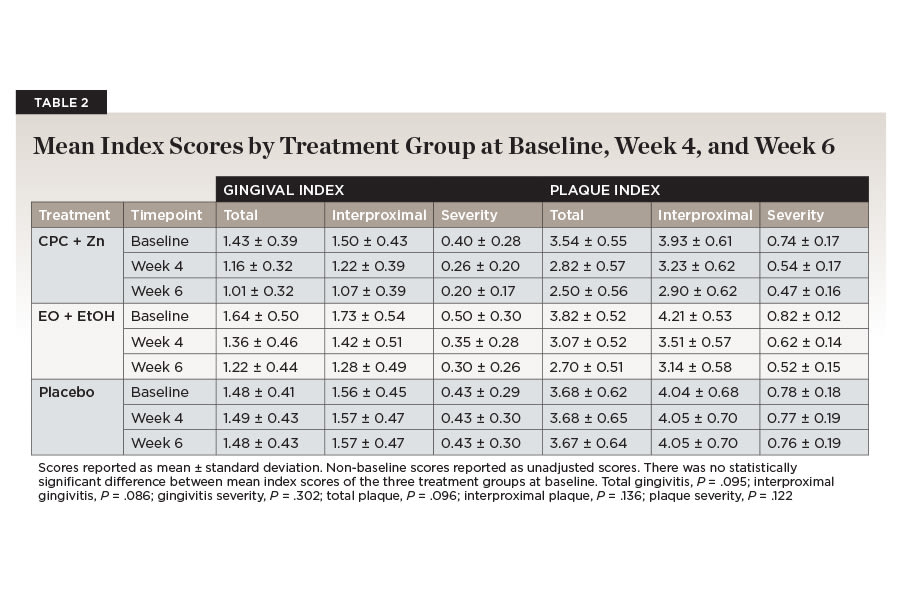
At baseline, there were no statistically significant (P > .05) differences between mean scores for any of the plaque or gingival indices between the three treatment groups (Table 2). For the placebo treatment group, there was no significant difference between mean plaque index or gingival index scores compared to baseline at either week 4 or week 6 (Table 3).
CPC + Zn Treatment Over Time Compared to Baseline
All plaque index scores decreased significantly over time for the CPC + Zn treatment group (Table 3). Total plaque scores were reduced by 30.2% from an average of 3.54 (±0.55) at baseline to 2.57 (±0.08) baseline-adjusted mean score by week 6 (P < .001). Interproximal plaque scores were reduced by 26.8% from an average of 3.93 (±0.61) at baseline to 2.97 (±0.09) baseline-adjusted mean score by week 6 (P < .001). Plaque severity was reduced by 37.2% from an average of 0.74 (±0.17) at baseline to 0.49 (±0.02) baseline-adjusted mean score by week 6 (P < .001). All mean plaque index scores showed a continuous reduction after 4 and 6 weeks of treatment with CPC + Zn mouthwash compared to baseline (Table 2).
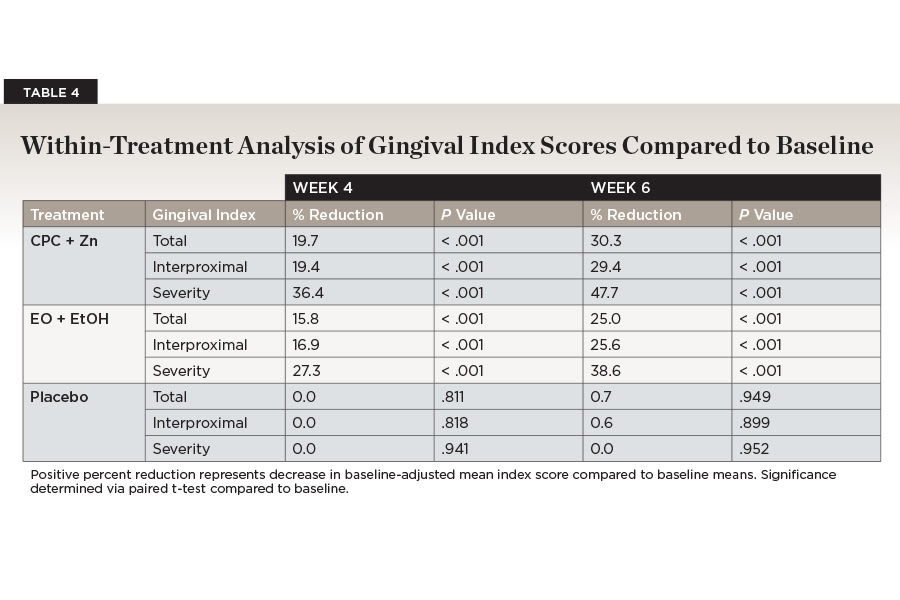
All gingival index scores decreased significantly over time for the CPC + Zn treatment group (Table 4). Total gingivitis was reduced by 30.3% from an average of 1.43 (±0.39) at baseline to 1.06 (±0.05) baseline-adjusted mean score by week 6 (P < .001). Interproximal gingivitis was reduced by 29.4% from an average of 1.50 (±0.43) at baseline to 1.13 (±0.06) baseline-adjusted mean score by week 6 (P < .001). Gingivitis severity was reduced by 47.7% from an average of 0.40 (±0.28) at baseline to 0.23 (±0.03) baseline-adjusted mean score by week 6 (P < .001). All mean gingival index scores showed a continuous reduction after 4 and 6 weeks of treatment with CPC + Zn mouthwash compared to baseline (Table 2).
EO + EtOH Treatment Over Time Compared to Baseline
All plaque index scores decreased significantly over time for the EO + EtOH treatment group (Table 3). Total plaque scores were reduced by 28.5% from an average of 3.82 (±0.52) at baseline to 2.63 (±0.08) baseline-adjusted mean score by week 6 (P < .001). Interproximal plaque scores were reduced by 24.6% from an average of 4.21 (±0.53) at baseline to 3.06 (±0.09) baseline-adjusted mean score by week 6 (P < .001). Plaque severity was reduced by 35.9% from an average of 0.82 (±0.12) at baseline to 0.50 (±0.02) baseline-adjusted mean score by week 6 (P < .001). All mean plaque index scores showed a continuous reduction after 4 and 6 weeks of treatment with EO + EtOH mouthwash compared to baseline (Table 2).
All gingival index scores decreased significantly over time for the EO + EtOH treatment group (Table 4). Total gingivitis was reduced by 25.0% from an average of 1.64 (±0.50) at baseline to 1.14 (±0.05) baseline-adjusted mean score by week 6 (P < .001). Interproximal gingivitis was reduced by 25.6% from an average of 1.73 (±0.54) at baseline to 1.19 (±0.06) baseline-adjusted mean score by week 6 (P < .001). Gingivitis severity was reduced by 38.6% from an average of 0.50 (±0.30) at baseline to 0.27 (±0.03) baseline-adjusted mean score by week 6 (P < .001). All mean gingival index scores showed a continuous reduction after 4 and 6 weeks of treatment with EO + EtOH mouthwash compared to baseline (Table 2).
Between-Treatment Analysis
CPC + Zn Treatment Compared to EO + EtOH Treatment
While both CPC + Zn and EO + EtOH treatment groups significantly decreased plaque over time, there was no significant difference between the groups’ percentage plaque reductions observed across any of the three plaque indices at either week 4 or week 6 (all: P > .05) (Table 5). Gingivitis was also significantly reduced in both the CPC + Zn and EO + EtOH treatment groups, but there was no significant difference between the two groups in percentage reduction observed across any of the gingival indices at either week 4 or week 6 (all: P ≥ .05) (Table 6).
CPC + Zn Treatment Compared to Placebo Treatment
The CPC + Zn treatment group had a 30.0% greater reduction in total plaque, a 26.8% greater reduction in interproximal plaque, and a 35.5% reduction in plaque severity after 6 weeks compared to the group that rinsed with a placebo mouthwash (all: P < .001) (Table 5).
This group also exhibited a significantly greater reduction in gingivitis across all indices. Clinicians observed a 29.8% greater reduction in total gingivitis, a 28.9% greater reduction in interproximal gingivitis, and a 47.7% reduction in gingivitis severity after 6 weeks of rinsing with CPC + Zn mouthwash compared to the group that rinsed with a placebo mouthwash (all: P < .001) (Table 6).
EO + EtOH Treatment Compared to Placebo Treatment
The EO + EtOH treatment group had a 28.3% greater reduction in total plaque, a 24.6% greater reduction in interproximal plaque, and a 34.2% reduction in plaque severity after 6 weeks of rinsing with EO + EtOH mouthwash compared to the group that rinsed with a placebo mouthwash (all: P < .001) (Table 5).
This group also exhibited a significantly greater reduction in gingivitis across all indices. Clinicians observed a 24.5% greater reduction in total gingivitis, a 25.2% greater reduction in interproximal gingivitis, and a 38.6% reduction in gingivitis severity after 6 weeks of rinsing with EO + EtOH mouthwash compared to the group that rinsed with a placebo mouthwash (all: P < .001) (Table 6).
Discussion
This 6-week clinical trial demonstrated parity between CPC + Zn and EO + EtOH mouthwash formulas in the reduction of dental plaque and gingivitis. Both treatment groups significantly reduced whole-mouth, interproximal, and severity plaque and gingivitis scores compared to baseline and the placebo. While there was no significant difference between the efficacy of these two mouthwashes compared to each other, CPC + Zn consistently outperformed EO + EtOH compared to placebo, as evidenced by a 47.7% greater reduction in gingivitis severity by CPC + Zn after 6 weeks of treatment compliance compared to a 38.6% greater reduction after EO + EtOH treatment.
A previous clinical trial featuring a CPC mouthwash without zinc lactate compared to EO + EtOH mouthwash also demonstrated no statistical difference between the treatments for all plaque and gingivitis indices measured after 6 weeks of use.26 Given that CPC + Zn has been reported to have a 54.5% greater reduction in gingivitis severity after 6 weeks of use compared to a mouthwash formula containing 0.07% CPC alone,13 there was reason to believe that the CPC + Zn formula would have a greater statistically significant reduction in plaque and gingivitis indices compared to EO + EtOH. Additionally, Schaeffer et al has shown in an in vitro biofilm study that CPC + Zn and EO + EtOH had a similar percent reduction in biofilm viability after 2 hours of mouthwash treatment compared to the negative control at 42.8% and 42.6% reduction, respectively.27 However, after 5 hours of mouthwash treatment the CPC + Zn formula resulted in a 62.1% reduction and the EO + EtOH declined to a 19.46% reduction, showing that the bacteria in biofilm increased in viability after prolonged exposure to EO + EtOH but decreased in viability after prolonged exposure to CPC + Zn. The reason that the clinical study reported here did not find a clinically significant difference between CPC + Zn and EO + EtOH despite CPC + Zn’s proven anti-biofilm properties could reflect that standard plaque indices do not distinguish between living and dead bacteria and may not correlate with the proportion of bacterial viability within the plaque biofilm.
Despite suggestions that the act of mouth rinsing alone can reduce plaque and gum inflammation,28 the placebo mouthwash in this trial demonstrated no reduction in total plaque and total gingivitis after 4 weeks and 6 weeks of use. The 30% greater reduction than placebo in whole-mouth plaque and gingivitis after 6 weeks of CPC + Zn treatment observed in this study is supported by two clinical studies comparing CPC + Zn mouthwash compared to mouthwash containing 0.02% sodium fluoride, which found that rinsing with CPC + Zn significantly decreased whole-mouth plaque and gingivitis by an average of 26.4% and 21.1% more than the control, respectively.29
Conclusion
This clinical trial evaluated the antiplaque and antigingivitis efficacy of a CPC + Zn mouthwash compared to an EO + EtOH mouthwash and a placebo mouthwash. It was hypothesized that both CPC + Zn and EO + EtOH would equally reduce plaque and gingivitis significantly compared to the placebo. The results of the trial fully supported this hypothesis. There was, however, no significant difference between the two test mouthwashes at the measured timepoints. Given that mouthwashes formulated with ethanol can be associated with intense oral pain and lower user compliance, an alcohol-free CPC + Zn mouthwash may be an effective alternative for reducing plaque and treating gingivitis in patients who prefer to avoid the oral pain associated with alcohol-containing mouthwashes.
ACKNOWLEDGMENTS
Technical writing was provided by Meghan A. Berryman, PhD. The author contributions were as follows: BS and BG: conceptualization, funding acquisition, supervision; RD: methodology; LM: formal analysis, validation; JN and AE: project administration, investigation. All authors contributed to writing, review, and editing.
DISCLOSURES
This clinical trial was supported by funding from Colgate-Palmolive Company. Institutional Review Board Approval: The study was reviewed and approved by Consejo Nacional de Bioética en Salud (CONABIOS) Av. Bolívar No. 902, Santo Domingo, República Dominicana, Dentro de la Universidad Católica Santo Domingo.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
ABOUT THE AUTHORS
Bernal Stewart, MSc
Director Clinical Research and Innovation, Colgate-Palmolive Co., Piscataway, New Jersey
Bayardo García-Godoy, DMD, MSc
Director Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
Rensl Dillon, MS, PhD
Former Director Research and Innovation, Colgate-Palmolive Co., Piscataway, New Jersey
Luis R. Mateo, MA
President, LRM Statistical Consulting LLC, West Orange, New Jersey
Joselyn Noboa, DDS
Dentist, Dental Research Associates, Santo Domingo, Dominican Republic
Augusto R. Elias-Boneta, DMD, MSD
Assistant Dean for Research, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico