A 62-year-old female patient with a history of type 1 diabetes mellitus, obesity, and hypertension developed fixed drug eruption following semaglutide administration, representing the first reported case of this reaction to the glucagon-like peptide-1 receptor agonist, according to researchers at Temple University Lewis Katz School of Medicine.
The patient developed characteristic lesions within 72 hours of initiating 2 mg of subcutaneous semaglutide injections weekly. According to the case report, published in JAAD Case Reports, researchers described the lesions as "initially erythematous and pruritic reactions [that] subsequently progressed into asymptomatic chronic hyperpigmented patches with an erythematous border."
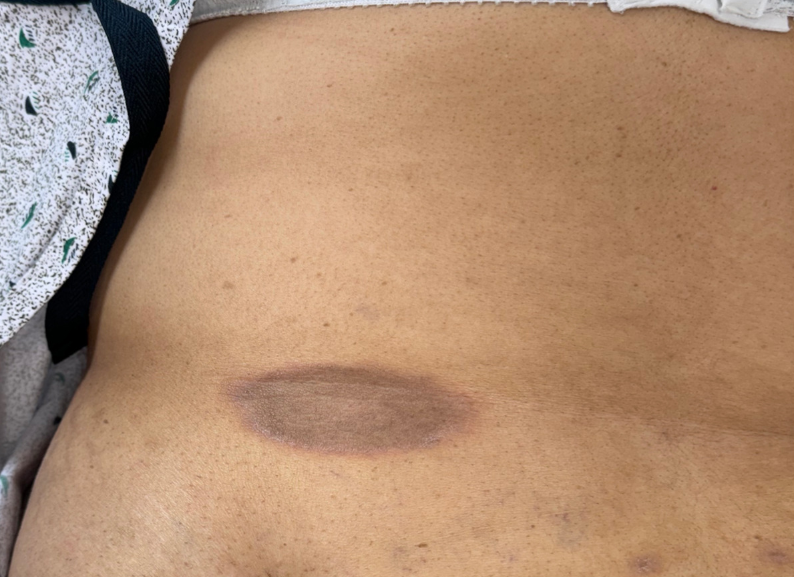
Physical examination revealed "multiple dusky hyperpigmented round patches with erythematous borders on her buttock and abdomen." A 4-mm punch biopsy from the right buttock demonstrated "lichenoid interface dermatitis ... [with] numerous necrotic keratinocytes ... throughout the epidermis with conspicuous pigment incontinence seen throughout the superficial dermis."
Lichenoid interface dermatitis with necrotic keratinocytes and pigment incontinence in the epidermis and superficial dermis (H&E 100×).
The case highlighted a distinct presentation from previously documented semaglutide reactions. The researchers noted that while drug-induced hypersensitivity reactions, including generalized pruritus and rash, have been documented, this presentation represented a delayed hypersensitivity reaction "characterized by well-defined, recurrent patches at discrete sites, driven by localized immune responses involving drug-specific cytotoxic T cells retained in the skin."
Following discontinuation of semaglutide, the patient showed complete resolution of active lesions at 1-month follow-up, with residual postinflammatory hyperpigmentation.
The researchers concluded that "maintaining a high index of suspicion and advising patients to report any unusual dermatologic reactions during treatment" with glucagon-like peptide-1 receptor agonists.
Full disclosures can be found in the case report.
