At the 2025 annual meeting of the Association for Research in Vision and Ophthalmology in Salt Lake City, data were presented from Alkeus Pharmaceuticals’ phase 2 SAGA clinical study of gildeuretinol for geographic atrophy (GA) secondary to age-related macular degeneration (AMD) in which patients treated with oral gildeuretinol (ALK-001) had a slower decline in VFQ-25 (vision-related quality of life) and FRI (functional reading independence) index scores compared to placebo at month 24.
Data Highlights From the SAGA Study
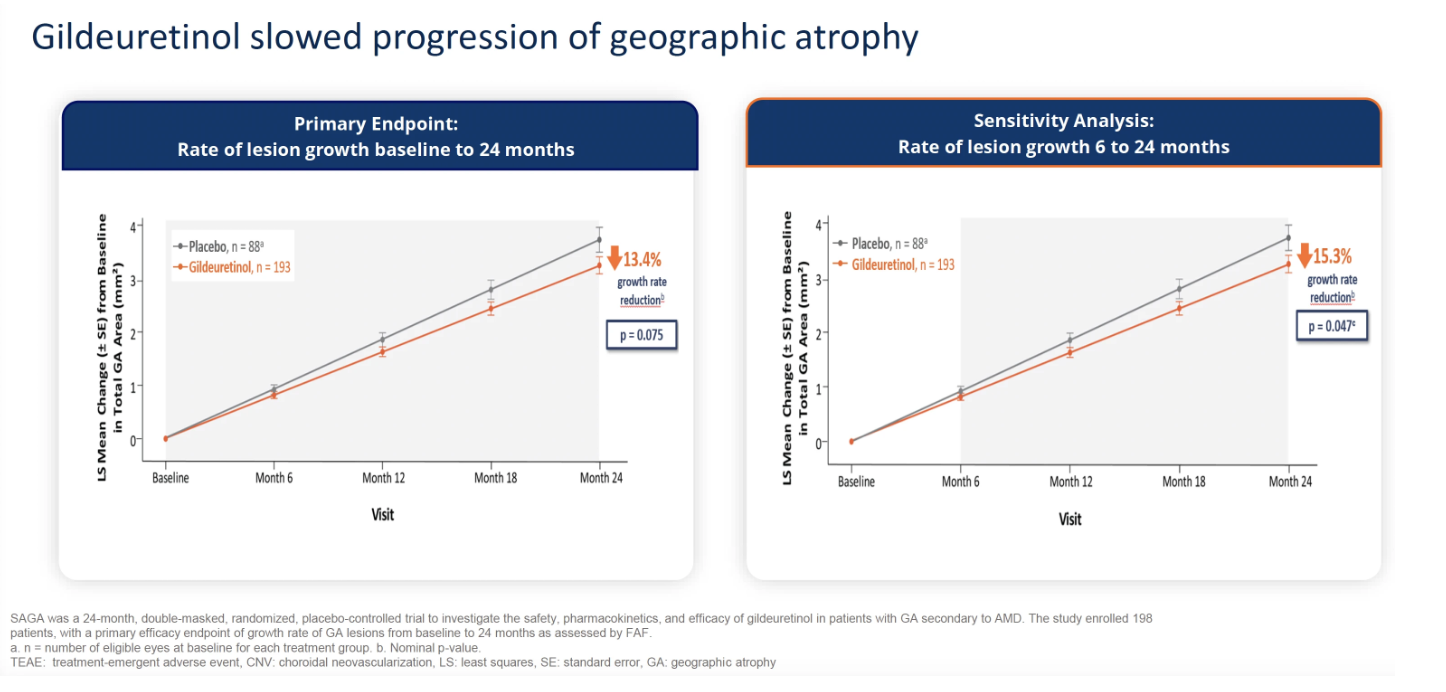
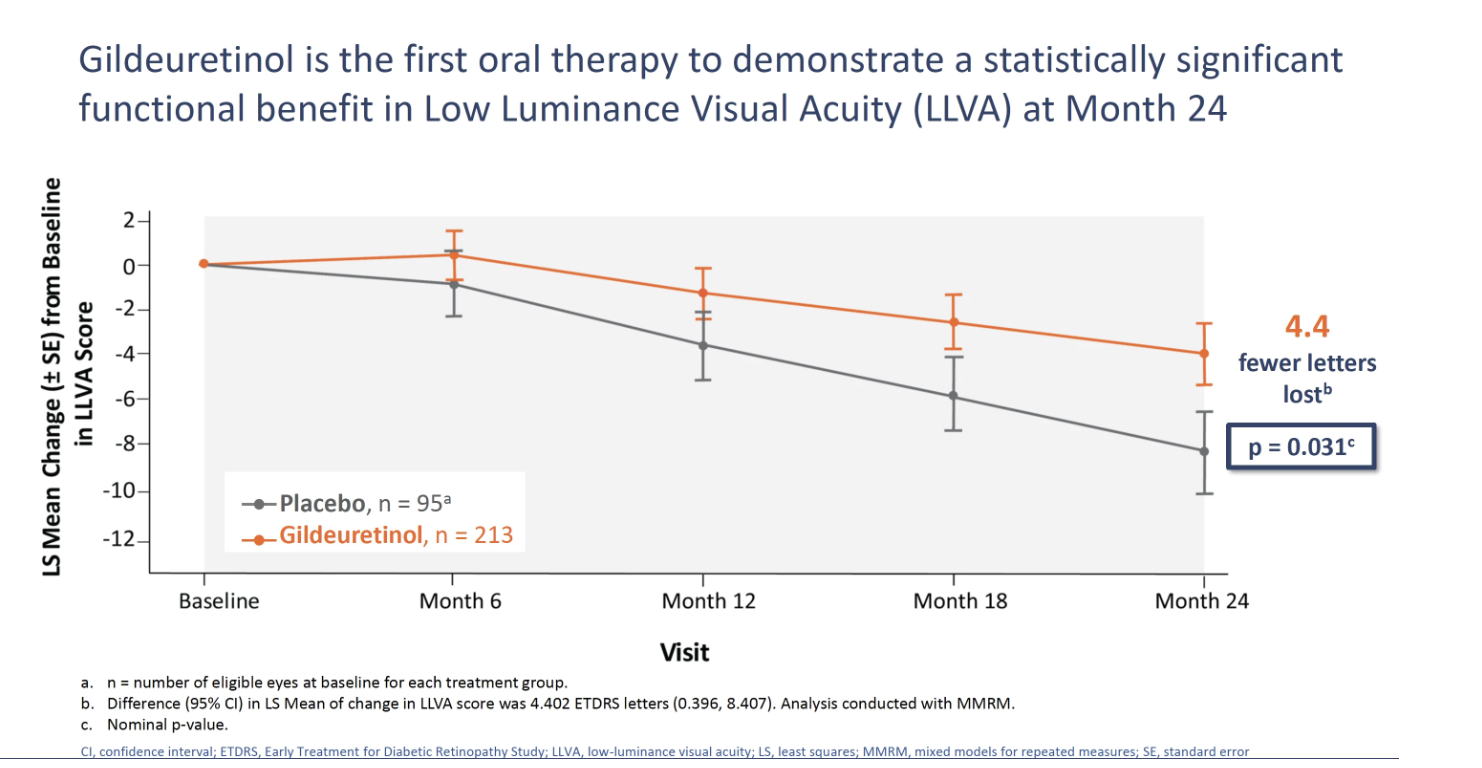
In the SAGA study, a placebo-controlled, double-masked, phase 2 study of gildeuretinol in GA secondary to AMD, gildeuretinol showed a clinically meaningful trend in slowing GA growth rate of 13.4% from 0 to 24 months (P=.075), the study’s primary endpoint, the company said in a news release. In a sensitivity analysis, gildeuretinol showed a statistically significant reduction in the GA lesion growth rate of 15.3% vs placebo from 6 to 24 months (P=.047). Gildeuretinol also demonstrated a statistically significant visual function improvement, showing 4.4 fewer letters lost (P=.031) in low luminance visual acuity (LLVA) over 24 months, the first key secondary endpoint.
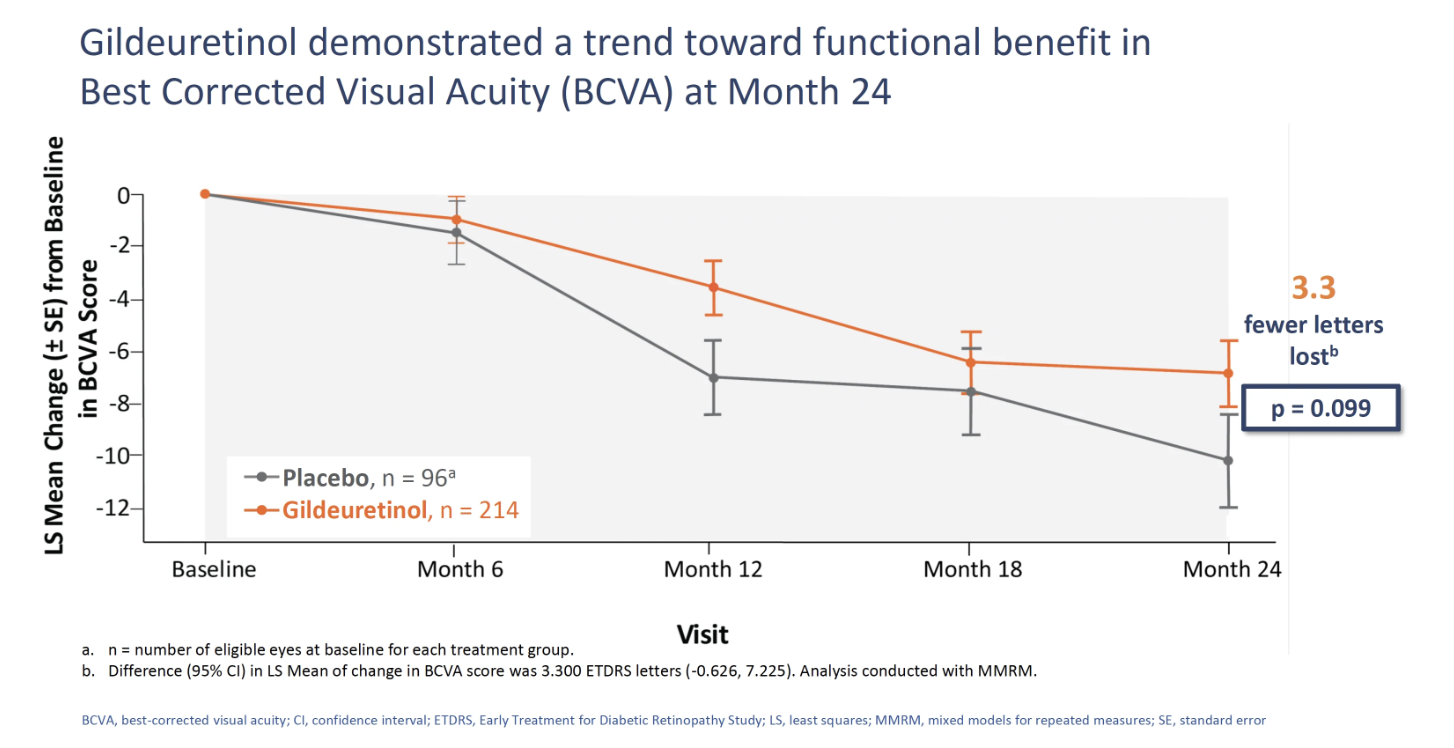
In addition, a trend toward functional benefit in best corrected visual acuity (BCVA) was demonstrated at 24 months with 3.3 fewer letters lost (P=.099), also a key secondary endpoint.
The majority of adverse events were mild or moderate. There was a lower rate of choroidal neovascularization-related adverse events in at-risk patients; all lab treatment-emergent adverse events were isolated events and none were sustained; there were no symptoms of hypervitaminosis A or hypovitaminosis A. Also, there were no reports of delayed dark adaptation or chromatopsia, vasculitis, intraocular inflammation, or endophthalmitis.
According to the company, decreasing vitamin A dimerization is a potential mechanism of action to treat GA. Compared with placebo, the results demonstrated a trend toward slower GA lesion growth rate from baseline to 24 months; statistically significant slower GA lesion growth rate from 6 to 24 months; and statistically significant visual function benefit in LLVA. According to the company, this is the first investigational oral therapy to demonstrate this. In addition, gildeuretinol was well tolerated and exhibited a favorable safety profile consistent with studies in the gildeuretinol Stargardt program.
“We are pleased with these results from the SAGA study which showed that gildeuretinol provides meaningful functional benefits to people living with GA,” said Seemi Khan, MD, MPH, MBA, chief medical officer of Alkeus Pharmaceuticals, and a member of the SAGA study team, in the news release. “GA secondary to AMD and Stargardt disease share a common pathophysiology, the accumulation of vitamin A dimers that are toxic to the retina. Importantly, these data, along with the positive results reported from our TEASE clinical program evaluating gildeuretinol for Stargardt disease, provide additional clinical evidence supporting the unique mechanism of gildeuretinol.”
David Boyer, MD, Retina-Vitreous Associates Medical Group in Los Angeles, who presented the study at ARVO, also noted: “For my patients living with GA secondary to AMD, the symptoms can have a profound impact on their daily activities and their independence. I’m very encouraged by these results which show meaningful trends in improvement when treated with investigational oral gildeuretinol. These results demonstrate that gildeuretinol should be further evaluated as a potential systemic treatment of GA secondary to AMD.”
Source: Ophthalmology Management
